Abstract
Introduction: Risk of venous thromboembolism (VTE) appears elevated in patients with lung cancer receiving immune checkpoint inhibitor (ICI) cancer therapy and may be associated with poor clinical outcomes. Little is known about specific risk factors predisposing to ICI-associated VTE for identifying a sufficiently high-risk group of outpatients for thromboprophylaxis. Therefore, we assessed risk factors for predicting ICI-associated VTE using baseline clinical and laboratory variables in patients with non-small cell lung cancer (NSCLC), representing the major solid tumor cancer type with several years of FDA approval of ICI therapy.
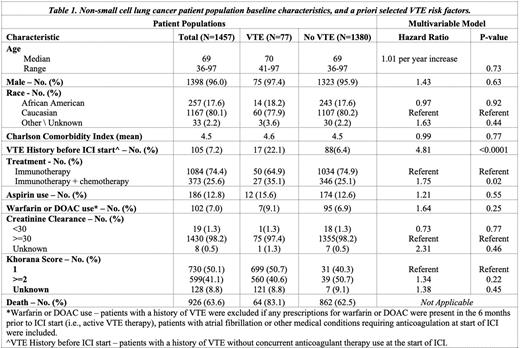
Methods: The association of VTE (PE or DVT) with a priori selected, baseline patient characteristics (Table 1) was characterized in outpatients with lung cancer diagnosed between 2015-2019 newly receiving ICI therapy in a retrospective, observational cohort study using the Veterans Affairs healthcare system (VA). Patients were ineligible if they had a history of VTE plus a prescription for anticoagulant therapy within 6 months prior to ICI start (N=150), to eliminate potential misclassification of these patients as new VTE events. Patients with anticoagulation or aspirin use for other medical conditions were eligible. A new VTE diagnosis was assessed starting 72 hours after ICI start (index date) until 6 months post index date. Patients with VTE within 72 hours of the ICI index date were excluded (N=12) to avoid including unrecognized VTE diagnoses present prior to ICI therapy for a total eligible cohort size of 1457 patients. VTE was defined using ICD 9/10 codes for either PE, DVT, or splanchnic VTE requiring anticoagulation, while excluding superficial VTE. Cancer therapy was categorized into ICI-only therapy (N=1073 single ICI; or N=11 dual ICI therapy) versus ICI-chemotherapy combinations (N=373). Patients receiving ICI in a therapy combination other than chemotherapy (N=2) were excluded. All potential risk factors were a priori selected (Table 1). The association between risk factors and VTE was assessed in a Fine-Gray competing risk model to adjust for the competing risk of death.
Results: 77 (5.3%) patients experienced a VTE by 6-months of whom 27 patients received ICI-chemotherapy and 50 ICI-only therapy. Distribution of VTE was as follows: PE 34 (44%), PE + DVT 8 (10%), proximal DVT 8 (10%), distal DVT 1 (1%), unspecified lower extremity DVT 14 (18%), upper extremity DVT 8 (10%), and other 4 (5%). Table 1 describes the patient characteristics by 6-month VTE status, as well as total patient population and a priori defined risk factors. Median age was 69 (range 36-97), 1,398 (96%) men, and the majority 1,167 (80.1%) Caucasian. A history of VTE prior to the ICI index date and without current anticoagulation use was observed in 105 (7.2%) of patients. During the 6-month study follow-up period, 926 (63.6%) of all patients died, including 83.1% of patients who developed VTE, and 62.5% who did not. The competing risk VTE multivariable modeling identified the following independent risk factors (Table 1): ICI-chemotherapy HR=1.75 (95% CI: 1.07-2.83) and history of VTE more than 6 months prior to ICI start HR= 4.81 (95% CI: 2.60-8.90). Race and comorbidities were not identified as independent risk factors. Khorana Score, as well as medical conditions requiring aspirin or anticoagulation use (DOAC, warfarin) were associated with a limited increased risk for VTE but did not reach statistical significance. Of the 102 patients on DOAC or warfarin at the start of ICI, 7 (9%) developed VTE. On review of these patients, 4 were off anticoagulation at the time they developed VTE.
Discussion: Using easily extractable, a priori identified, baseline characteristics from patients with lung cancer, we identified the following risk factors for ICI-associated VTE that are independent from the Khorana Score: ICI-chemotherapy combination therapy, as well as a distant history of VTE at least 6 months prior to the ICI index date. A follow-up model will further assess metastatic disease, recent hospitalization, frailty, and surgery. As the diversity of cancer-directed therapy increases, modifications to available VTE risk assessment models could improve personalized thromboprophylaxis strategies to decrease risk of cancer-related VTE in high-risk populations. A risk assessment model for VTE in these patients is under development to formally assess all proposed risk factors.
Disclosures
Sanfilippo:ACS-IRG: Research Funding; Covington & Burling LLP: Other: Expert Case Review; Health Services Advisory Group: Consultancy; K01 NHLBI: Research Funding; NHLBI NIH: Other: Loan Repayment program; Quinn Johnston: Other: Expert Case Review. Lyman:Squibb: Consultancy; Sandoz: Consultancy; Seattle Genetics: Consultancy; Fresenius Kabi: Consultancy; Merck: Consultancy; Samsung Bioepis: Consultancy; G1 Therapeutics: Consultancy; Amgen: Research Funding; Beyond Spring: Consultancy. Kuderer:Bristol Myers Squibb: Consultancy; Beyond Spring: Consultancy; Sandoz: Consultancy; G1 Therapeutics: Consultancy; Seattle Genetics: Consultancy; Janssen: Consultancy; Pfizer: Consultancy; Spectrum: Consultancy; Amgen: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal